Clean Gear Matters
In recent years, cancer in the fire service has become a topic of growing concern, as numerous studies have highlighted firefighters’ increased risk for certain cancers. Several numbers appear in fire service discussions regarding this increased risk; however, the most reliable data were published by the National Institute for Occupational Safety and Health (NIOSH) in 2014. This scientific research found a 14 percent increase in all cancer deaths for firefighters1 relative to the general population rate. This increase in risk for cancer deaths may be, at least partially, explained by firefighters’ occupational exposure to toxic chemicals.
During fire responses, firefighters are routinely exposed to smoke (particulate and gas) that contains a variety of chemicals, including polycyclic aromatic hydrocarbons (PAHs) and flame retardants (FRs). Exposure to PAHs are especially concerning because some PAHs are known human carcinogens. In a study conducted by Illinois Fire Service Institute (IFSI) and NIOSH, elevated levels of PAH metabolites were identified in firefighters’ urine after a live-fire scenario even though SCBA were used throughout, suggesting PAHs may be absorbed through the skin.2
It has been hypothesized that FRs in household products may be released into the environment when they burn and could also present an exposure hazard for firefighters. Some FRs, like the phased-out polybrominated diphenyl ethers (PBDEs), have been associated with altered hormone regulation.3 Some organophosphate flame retardants (OPFRs) have been associated with cell toxicity,4 and a few non-PBDE brominated flame retardants (NPBFRs) have been observed to be endocrine disruptors (chemicals that may interfere with the hormone systems and can produce developmental, reproductive, neurological, and immune effects).5 Recent studies have found elevated levels of brominated and organophosphate flame retardants in firefighters’ bodies compared to general population levels.6
Firefighter hood contamination
PPE, including protective hoods, helps reduce firefighters’ exposure to these toxic substances by reducing skin exposure during a fire response. Protective hoods are characterized by the NFPA as the interface element of the protective ensemble that provides limited protection to the coat/helmet/SCBA face-piece interface area.7 This PPE ensemble element is in direct contact with neck and face skin, which is thinner and tends to be more absorptive than skin on most other parts of the body.
Previous studies have characterized chemical exposures on the neck following firefighting. It is often assumed that most of this exposure happened during the firefight (via penetration of chemicals through or around the hoods). However, if hoods are worn for multiple responses, there is a possibility that the hood itself will retain fireground products of combustion and be a source of chemical exposure. This dirt or soot has the potential to contain PAHs, FRs and other compounds. Thus, over time, hoods with residual contamination may contribute to firefighters’ overall exposure to dangerous chemicals.
Laundering sock hoods
Historically, protective hoods may have been worn for multiple responses without laundering. However, over the past few years, many fire departments have implemented hood exchange and/or laundering programs in an attempt to reduce potential exposure through contaminated hoods. This is a positive step for the fire service and a reasonable solution in light of available evidence. However, we are unaware of any data that tells us just how effective laundering is at removing contamination on hoods, or if there are any unintended consequences of this action. In other industries, it has been suggested that cleaning efficiency will depend on the solubility (fat-soluble compounds are harder to clean) and molecular weight (heavier compounds are harder to clean) of the contaminants.
To address this knowledge gap in the fire service, we set out to determine the effectiveness of laundering to reduce or remove PAHs and FRs from sock hoods. PAHs are fat-soluble but generally lighter than FRs. Brominated FRs are fat-soluble (and persistent in the environment), while OPFRs tend to be water-soluble. So there are materials with a range of different properties that we want to remove in a single-wash cycle.
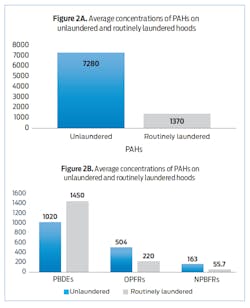
In a collaborative effort between IFSI, NIOSH and Underwriter Laboratories (UL) Firefighter Safety Research Institute (FSRI), we asked 10 firefighters, who were paired, to complete different fireground job assignments while responding to realistic fire scenarios. For each pair of firefighters, one hood was routinely laundered after every scenario and one was not. The hoods that were cleaned were laundered together in a washing machine following manufacturer and NFPA guidelines. In total, each firefighter responded to four scenarios. At the end of the study, we took samples from five routinely laundered and five unlaundered hoods and analyzed them for PAHs and three classes of FRs—PBDEs, NPBFRs and OPFRs—that were on the surface and embedded in the hoods themselves.8
Surprisingly, PBDEs were almost 50 percent higher in the laundered hoods compared to the unlaundered hoods (Figure 2B). A previous study had indicated poor extraction of PBDEs contaminating polyester fabrics into laundry water.9 Thus, we were not surprised to find significant residual PBDE contamination on laundered hoods, but we were surprised to find higher contamination. Where did the additional PBDE contamination come from? Because of this finding, we conducted a follow-on study to examine cross-contamination during laundering.
Cross-contamination study
For our follow-on study, we laundered brand new, unexposed hoods and a set of exposed hoods similar to those used in Figure 2 along with a set of heavily contaminated hoods (containing relatively high levels of PAHs and FRs). Altogether, 12 hoods were laundered at one time in a load of laundry representative of what may be done at a fire department where all hoods are gathered and laundered after a structure fire response regardless of contamination level.
After laundering, we collected and analyzed samples from the hoods to evaluate differences before and after laundering. Interestingly, all hoods that were brand new—and had no PBDE, NPBFR or OPFR contamination before laundering—had measurable contamination after laundering (Figure 3). This finding clearly indicates there can be cross-contamination of FRs between hoods during the laundering process. Additionally, some previously exposed hood samples had even higher PBDE levels after laundering than before, corroborating our earlier findings.
PAH concentrations were low on the brand new hoods after laundering, and PAH contamination on the exposed hoods were significantly reduced after laundering. This finding is of particular importance because PAHs are the only known human carcinogens in the class of chemicals analyzed in this study. Overall, cross-contamination of hoods during laundering appears to occur for FRs, and is less of a concern for PAHs.
Ongoing sweat extraction study
To begin addressing these remaining questions, we have embarked on a study to determine the amount of residual contamination on hoods that can be extracted in synthetic sweat solution. Importantly, the sweat solution contains both water and lipids (fats) and other properties that are characteristic of actual human sweat. Routinely laundered hood samples from our previous study will be placed in the sweat solution for a period that represents how long firefighters may wear hoods before, during and after an emergency fire response. Hood and sweat samples will then be analyzed for a variety of FRs. This experiment will increase our understanding of how hood contamination relates to dermal exposure and potential for absorption.
Implications for other firefighter PPE
Our findings may have implications for other components of the turnout gear ensemble. If chemicals are capable of cross-contaminating hoods during laundering, then cross-contamination may also happen during the laundering of turnout jackets or trousers, gloves or helmet liners. It is possible that some contamination on exterior materials could transfer to interior materials that are in direct contact with the skin if outer shells and liners are not separated during laundering as recommended by NFPA and others. Certainly, further research is needed. Studies are underway by fellow researchers to determine “How Clean Is Clean?”
Further, NIOSH, IFSI, and UL recently completed the field experiments for the “PPE Cleaning Study” to examine residual contamination with repeated laundering. Importantly, this study also aims to understand how repeated laundering affects the protective properties of turnout gear, including tear resistance, flame resistance and thermal protection performance. These studies, as well as the aforementioned sweat-extraction study, should provide further insight into how laundering firefighter PPE impacts firefighters’ potential exposure to chemicals. These results, along with previous research projects, will be made available to the fire service through our websites at cdc.gov/niosh/firefighters and fsi.illinois.edu/CardioChemRisks.
Final thoughts
While the potential for cross-contamination of FRs during laundering is something that should be explored further, the preponderance of evidence suggests that it is still prudent to launder contaminated hoods after fire responses to reduce the potential for exposure upon subsequent use. Based on our study findings, laundering will remove a large proportion of the PAHs from hoods, and it is likely that other hazardous or potentially carcinogenic compounds will also be removed. Also important are other measures to minimize skin exposure, such as careful removal of hoods and gloves to limit the amount that transfers to the skin (see videos at youtube.com/watch?v=QyAt5WHf5uM and youtube.com/watch?v=9uYp0ZQP158), on-scene cleaning of skin (e.g., skin cleansing wipes), and showering as soon as possible at the station.
Sidebar: Key Take-Home Messages
- Laundering hoods is effective at removing a large portion of PAH contamination, which is by far the most abundant contaminant found on the hoods and is an important part of firefighter hygiene and PPE cleaning.
- Consider segregating firefighter hoods by contamination level to reduce the potential for cross-contamination. If a firefighter responds to a call but is exposed to low levels of contamination compared to the rest of the crew, it may be beneficial to avoid washing with other crewmembers’ hoods.
- Do not launder hoods with base layers or station wear to reduce the risk for cross-contamination to these pieces of clothing that may directly contact skin.
- This study suggests the possibility that some contamination on turnout gear outer shells might transfer to inner liner materials that are in direct contact with the skin if they are not separated during laundering as recommended by NFPA and others.
References
1. Daniels, R.D., et al., Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950-2009). Occup Environ Med, 2014. 71(6): p. 388-97.
2. Fent, K.W., Eisenberg, J., Snawder, J., Sammons, D., Pleil, J., Stiegel, M., Mueller, C., Horn, G., Dalton, J, Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure FIres. The Annals of Occupational Hygiene, 2014. 58(7): p. 830-845.
3. Linares, V., M. Belles, and J.L. Domingo, Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol, 2015.
4. Behl, M., Rice, J.R., Smith, M.V., et al, Editor's Highlight: Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphneyl Ethers to Caenorhabditiselegans. Toxicological Sciences, 2016. 154(2): p. 241-252.
5. Saunders, D.M., Hagley, E.B., Hecker, M., Mankidy, R., Giesy, J.P. , In Vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, and TBCO. Chemosphere, 2013. 91: p. 1386-1394.
6. Jayatilaka, N.K., et al., Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem, 2017. 409(5): p. 1323-1332.
7. National Fire Protection Association (NFPA). Standard on Protective Ensembles for Structural Fire Fighting and Proximity Fire Fighting (NFPA 1971). [Standard]
8. (Submitted) Mayer, A., et al. Firefighter Hood Contamination: Efficiency of Laundering to Remove PAHs and FRs. Journal of Occupational and Environmental Hygiene, 2018.
9. Saini, A., C. Thaysen, L. Jantunen, R.H. Mcqueen, and M.L. Diamond. From Clothing to Laundry Water: Investigating the Fate of Phthalates, Brominated Flame Retardants, and Organophosphate Esters. Environmental Science & Technology, 50(17), 9289-9297 (2016).
Kenneth W. Fent is with the Division of Surveillance, Hazard Evaluations, and Field Studies with the National Institute for Occupational Safety and Health (NIOSH). Alexander C. Mayer is with RCS Corporation. Gavin P. Horn is with the Illinois Fire Service Institute (IFSI), University of Illinois at Urbana-Champaign. Denise L. Smith is with the Department of Health and Human Physiological Sciences at Skidmore College. Steve Kerber is with the UL Firefighter Safety Research Institute (FSRI).
Funding: This work was supported by the Department of Homeland Security Fire Prevention and Safety Grants ##EMW-2013-FP-00766 and #EMW-2015-FP-00646.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC). Mention of any company name or product does not constitute endorsement by NIOSH/CDC.
More from the Cancer Awareness & Prevention supplement:
- Fire Apparatus & Cancer Prevention
- Skellefteå Model Develops Healthy Firefighters
- Cancer Prevention Methods: Fact vs. Fiction
Download the full Cancer Awareness & Prevention supplement here.
About the Author
Dr. Kenneth Fent
Dr. Kenneth Fent is a research industrial hygienist at the National Institute for Occupational Safety and Health (NIOSH). Much of his research has focused on characterizing firefighters’ exposures to chemical agents, including carcinogens, and evaluating the effectiveness of practices intended to reduce exposures.
Dr. Denise Smith
Dr. Denise Smith is a professor at Skidmore College and a research scientist at the Illinois Fire Service Institute (IFSI). She conducts research on the heat stress and cardiovascular strain associated with firefighting, pathoanatomic cause of firefighter fatalities, and strategies to increase performance and decrease cardiovascular events in the fire service.
Stephen Kerber
Stephen Kerber is the director of the UL Firefighter Safety Research Institute (FSRI). He has led fire service research and education in the areas of ventilation, structural collapse and fire dynamics.