Smoke Burns
Firefighters tend to focus on visible fire (like a moth to a candle). Smoke is toxic and makes it hard to see, but does not present much of a threat to a firefighter wearing protective clothing and self-contained breathing apparatus, or does it?
Smoke
Smoke is a visible product of incomplete combustion. But what exactly is it? Smoke is a complex aerosol. An aerosol is a mixture of a gas and/or vapor and liquid or solid particulates. Key constituents of smoke include gases such as carbon monoxide and hydrogen cyanide, formaldehyde and benzene vapor, carbon particulate, and unburned pyrolysis products. Most of these components of smoke are toxic and flammable.
How can flammable gases and vapors exist inside a fire compartment? Why doesn't the fire consume them? The answer to these questions goes back to the fire triangle. Combustion requires fuel, oxygen, and heat in the correct proportion. Fuel and oxygen must be within the explosive or flammable range in order for combustion to occur (see Figure 2)
The Lower Explosive or Flammable Limit (LEL/LFL) is the minimum concentration of fuel vapor in air that will support combustion. Below this level, there is insufficient fuel for combustion to occur. The Upper Explosive or Flammable Limit (UEL/UFL) is the highest concentration of fuel gas or vapor in air that will support combustion. Above this level there is insufficient oxygen to support combustion. The flammable range falls between the LEL/LFL and UEL/UFL.
During the incipient stage of a compartment fire, adequate oxygen is available and fire development is predominantly limited by fuel characteristics and configuration. The developing fire consumes most of the pyrolysis products given off by solid fuels. However as the oxygen concentration in the compartment drops, pyrolysis products that are not consumed by the fire (excess pyrolyzate) and flammable products of incomplete combustion (i.e. carbon monoxide) begin to accumulate within the compartment.
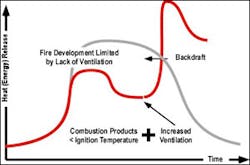
At some point in the combustion process within a closed compartment or compartment with limited ventilation the fire will be limited in its growth by the available oxygen supply. At this point the rate of heat release will stabilize and if ventilation is extremely limited will decline as illustrated in Figure 3.
It is important to point out that while the example in figure 3 illustrates a transition from fuel controlled to ventilation controlled prior to flashover, this is entirely dependent on the ventilation profile of the compartment. In other cases, the fire may reach flashover in the compartment of origin before becoming significantly ventilation controlled. Even though the rate of heat release slows when the fire becomes ventilation controlled, the temperature in the compartment can remain quite high. Radiant and convected heat from the fire can result in ongoing pyrolysis and further accumulation of pyrolysis products and flammable products of combustion within the compartment.
On a summer afternoon in 2005 Gresham Fire and Emergency Services responded to a fire in a doublewide mobile home. On arrival, smoke was pushing from windows on the Alpha and Bravo sides of the structure at moderate velocity. The windows were all darkly stained with condensed pyrolizate. After making entry through a doorway on Side Bravo, the crew on the first hoseline encountered little flaming combustion but substantial heat. Brief application of water to cool the hot gas layer and direct attack on the burning fuel quickly extinguished the fire. However, examination of the structure and its contents after the fire was extinguished illustrates the impact of radiant and convected heat in ventilation controlled fires (see Figure 4). Even though limited ventilation slowed fire development, the temperature in this compartment was high. Note the melted plastic on the microwave just to the left of center in the photo as well as pyrolysis and charring of the tops of the chair and couch. When firefighters arrived, this residence was full of hot, fuel rich smoke. The only thing missing was a source of air.
Most fires that progress beyond the incipient stage are ventilation controlled at the point where the fire department arrives. This means that if the ventilation profile changes to increase ventilation (i.e., a window fails or firefighters make an opening for ventilation and/or access); the fire can increase in intensity.
Hazards of Ventilation Controlled Fires
Graphic Under ventilation controlled conditions excess pyrolizate and flammable products of combustion present in smoke are a significant hazard to firefighters. Lets go back to the fire triangle (see Figure 4) to examine the nature of this threat. While fuel, heat, and oxygen are present in proportion to support combustion where the fire is burning, the heat of the fire is pyrolyzing more fuel vapor than the fire can consume. In addition, incomplete combustion results in production of flammable gases such as carbon monoxide. The speed of fire development is limited by the availability of atmospheric oxygen provided by the current ventilation profile of the compartment or building.
Firefighters generally think of ventilation as a life safety or fire control strategy or in terms of the specific tactics used to accomplish these strategies. However, buildings are always ventilated (to one extent or another). If this was not the case, the occupants could consume the atmospheric oxygen and make the space uninhabitable. Ventilation is the exchange of the atmosphere inside the structure with outside air. The term ventilation profile refers to the actual and potential ventilation of a structure based on structural openings, construction methods, and building ventilation systems at the present time (before or during fire conditions).
Under fire conditions, the ventilation profile may change in a variety of ways. The fire may cause structural materials such as window glazing to fail, increasing ventilation. Occupants exiting the structure may either close doors (reducing ventilation) or leave them open (increasing ventilation). These of changes are called unplanned ventilation. Firefighters can also influence ventilation by simply making entry (opening a door for access creates a ventilation opening) or by specific tactical action to change the ventilation profile. When ventilation is changed intentionally as part of the incident action plan, this is tactical ventilation.
When a compartment fire is ventilation controlled, what happens when the ventilation profile changes? Smoke contains a large quantity of unburned pyrolizate and flammable products of incomplete combustion as illustrated in Figure 5. Increased ventilation provides the oxygen necessary for fire development to increase and for the fire to quickly extend to the readily available fuel present in smoke. This can lead to one of two extreme fire behavior phenomenon; ventilation induced flashover or backdraft.
In a backdraft the smoke is above its ignition temperature and simply needs sufficient air to come into the flammable range for ignition to occur. However, the smoke is not above its ignition temperature, an external source of heat is required. In this case increased ventilation allows a rapid increase in combustion and transition to a fully developed fire (flashover). This process of ventilation induced flashover is illustrated in Figure 6.
It is important to note that this is not a backdraft. In a backdraft the fuel gas and vapor in the smoke is above its ignition temperature and the rate of combustion is generally much faster (deflagration) producing a more violent reaction as illustrated (see Figure 7).
While these two phenomenon are different, both present a significant threat to firefighters. Rapid fire progress due to ventilation induced flashover or backdraft is not an instantaneous process. Depending on a number of variables such as the location of the fire, current level of involvement, temperature of the smoke (hot gas) layer, and extent of the increase in ventilation these rapid fire progress phenomenon may take some time to occur. However, when it does, fire development will be extremely rapid! Firefighters entering a compartment or building containing an under ventilated fire must be aware of and manage the hazards presented by the potential for rapid fire progress. Remember, many if not most fires that have progressed beyond the incipient stage before firefighters arrival are ventilation controlled and present the potential for rapid fire progress with increased ventilation.
Controlling the Hazard
One of the major hazards presented by ventilation controlled fires is the potential for rapid fire progress when ventilation is increased as firefighters make entry for fire control and search operations. Recognition of this hazard is only the first step in reducing the risk of firefighting operations. Extinguishing the fire reduces the threat to both firefighters and the buildings occupants. However, this generally requires entering the building and locating the fire. When hot smoke is overhead, this is a high risk operation. Two basic strategies are used to control the risk presented by operating below hot, fuel rich smoke. Cooling the hot gas layer to prevent ignition and ventilation to remove the smoke from the compartment or building.
Subsequent articles will examine key fire behavior indicators related to potential for extreme fire behavior and the use of gas cooling and ventilation to control the fire environment and increase firefighter and occupant safety.
Study and Discussion Questions Reading about fire behavior is considerably different than experiencing it first hand. Resist the temptation to brush off basic concepts as too simple or elementary or more detailed explanations as too complex. Making a connection between theory and your own experience or the experiences of others is an effective way to learn. Use these questions to focus your thinking on how basic fire behavior theory connects with incidents that you or other members of your crew have responded to.
2. What has to happen for the fire to ignite fuel rich smoke that is above the upper flammable limit (lots of fuel and not enough oxygen)? How can this occur during firefighting operations?
Application Activity
At your next fire, take a few minutes after the fire has been extinguished to examine the building and its contents. Is evidence of pyrolysis present (look for melted plastic, and partially charred materials)? What smoke conditions did you encounter upon making entry? Do you think that excess (unburned) pyrolizate was present in the hot smoke layer? How did fire behavior change after you made entry or initiated ventilation? Why or why not?
References
- International Fire Service Training Association. (1998). Essentials of Firefighting (4th ed). Stillwater OK: Fire Protection Publications
- Karlsson, B. & Quintiere, J.G. (2000). Enclosure fire dynamics. Boca Raton, FL: CRC Press.
- Drysdale, D. (2000). An introduction to fire dynamics. Chichester, England: John Wiley & Sons.
- Grimwood, P., Hartin, E., McDonough, J., & Raffel, S. (2005). 3D firefighting: Training , techniques, and tactics. Stillwater, OK: Fire Protection Publications.