The Street Chemist - Part 24
A term often associated with hazardous materials and water is miscibility. If a chemical is miscible with water, it will mix with water, which could make clean up very difficult.
This is a very abbreviated explanation of polymers and plastics, which are fairly complicated subjects. An entire book could be written on them. The most important thing for responders to understand about monomers and polymers is to be able to recognize them and the danger they present in the uncontrolled conditions of the incident scene.
SPECIFIC GRAVITY
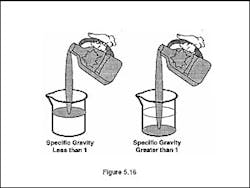
Specific gravity is to water what vapor density is to air. Specific gravity is the relationship of the weight of a liquid to water or another liquid (Figure 5.16). Like air, water is given a weight value of 1.
If a flammable liquid has a specific gravity greater than 1, it is heavier than water and will sink to the bottom in a water spill. If a flammable liquid has a specific gravity less than 1, it will float on top of water. The specific gravity of a flammable liquid is very important in a water spill because it will determine what tactics are necessary to contain the spill.
Specific gravity is the theory behind the construction of overflow and underflow dams, which are used to stop the flow of hazardous materials in water spills. Overflow dams are constructed for liquids heavier than water. The liquid sinks to the bottom of the water, and the water flows over the dam, while the hazardous liquid is stopped by the dam. Underflow dams are constructed for liquids lighter than water. The liquid floats on the surface of the water and is stopped by the top of the dam, while the water continues to flow through a pipe at the bottom of the dam.
A term often associated with hazardous materials and water is miscibility. If a chemical is miscible with water, it will mix with water, which could make clean up very difficult. If the chemical is not miscible with water, it will form a separate layer. The layer will form on top or on the bottom of the water, depending on the specific gravity of the liquid. Most flammable liquids are lighter than water and immiscible, so they float on the surface.
POLYMERIZATION/PLASTICS
Flammable liquids may undergo a chemical reaction called polymerization, in which a large number of simple molecules called monomers combine to form long-chained molecule called a polymer. This process is used under controlled conditions to create plastics (Figure 5.17). Alkene hydrocarbon compounds and hydrocarbon derivatives, such as aldehydes, alkyl halides, and esters, and the aromatic hydrocarbon styrene may undergo polymerization. There are other monomers that are flammable and can polymerize, but their primary hazard is poison. Monomers can be flammable liquids, flammable gases, and poisons.
When a monomer such as styrene is transported or stored, an inhibitor is included in solution to keep the styrene from polymerizing. An inhibitor, usually an organic compound, retards or stops an unwanted polymerization reaction. If an accident should occur, this inhibitor can become separated from the monomer and a runaway polymerization may occur. Phenol is used as an inhibitor for vinyl chloride. Phenol by the way is a deadly poison! Dibutylamine is used as an inhibitor for butadiene.
During a normal chemical reaction to create a particular polymer from a monomer, a catalyst is used to control the reaction. A catalyst is any substance that in a small amount noticeably affects the rate of a chemical reaction, without itself being consumed or undergoing a chemical change. For example, phosphoric acid is used as a catalyst in some polymerization reactions. Once an uncontrolled polymerization starts at an incident scene, it will not be stopped until it has completed its reaction, no matter what responders may try to do. If the polymerization occurs inside a tank, the tank may rupture violently. If a container of a monomer is exposed to fire, it is important to keep the container cool. Heat from an exposure fire may start the polymerization reaction. In the following example, the monomer vinyl chloride is shown along with the process of polymerization of the vinyl chloride molecules. (Figure 5.18)