Research Corner: Water Application During Suppression
During my education as a firefighter, I was taught to be cognizant of the steam produced when applying water inside a burning structure. Water application could create massive amounts of steam. If I wasn’t careful, water application could cause injuries to me, my fellow firefighters and even potentially trapped occupants. Was this concern, emphasized throughout my fire service education, rooted in science?
Testing a fire service concern
In 2013, the Underwriters Laboratories Firefighter Safety Research Institute (UL FSRI) was awarded a grant to evaluate the “Impact of Fire Attack Utilizing Interior and Exterior Streams on Firefighter Safety and Occupant Survival.” One of the goals of the project was to “develop and implement a methodology to measure moisture content in the modern fire environment to answer fire service concerns.” The idea that water application could increase the water vapor in the environment, resulting in firefighter injuries and further injury to trapped occupants, was highlighted as a significant concern of the U.S. fire service. To address this concern, UL FSRI partnered with the Illinois Fire Service Institute (IFSI) to develop a method of measuring water vapor in the fire environment using lasers designed to measure combustion gases in engine exhaust. The IFSI team utilized absorption spectrometry to conduct exploratory measurement of water vapor in a single-family structure test prop.
The 1,620-square-foot single-story test prop was designed to represent a single-story ranch home. The right side of the prop had a living room and kitchen. The four bedrooms were accessed down a long hallway. Three of the bedroom doors were open and one closed.
The experiments were broken into three ventilation cases: No Vent, Single Vent and Two Vent. Varying fire suppression methods were examined using each ventilation case to identify trends in effectiveness. In each of the ventilation cases, water vapor was measured in the open bedroom down the hall from the bedroom fires (Figure 1).
To further understand the results of the measurements, let’s look at the differences between water vapor and steam. Water vapor is the gaseous state of water. The percent concentration of water vapor by volume is unchanged by temperature. One way of reporting water vapor is relative humidity, or the percentage of water vapor in air relative to the maximum potential water vapor the air can hold, up to the boiling point of water or 212 degrees F (100 degrees C). The higher the air temperature, the more water vapor the air can hold.
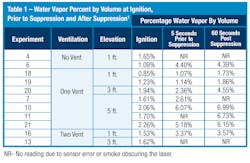
Steam is one type of water vapor, which is created when water is heated to its boiling point—212 degrees F (100 degrees C). Since steam is only created when water vapor is above 212 degrees F (100 degrees C), steam cannot be reported as relative humidity. This would offer an incomplete picture of the water vapor present. Instead, utilizing the percentage of water vapor in the gas by volume allows for a more direct comparison. Table 1 summarizes the results of the 11 experiments where data was collected in percent by volume of water vapor at ignition, five seconds prior to suppression and 60 seconds after suppression, where suppression was conducted utilizing a 150–165 gpm handline.
In almost all the experiments, the percent by volume of water vapor increased significantly after ignition, as shown by the values measured five seconds prior to the start of suppression. The higher the elevation the water vapor was measured, the more water vapor was detected. At elevations in the smoke layer (five feet), water vapor increased by two to four times the value measured at ignition prior to the application of any suppression water.
This increase in water vapor is likely from the combustion of the fuels within the structure.
What does this mean?
To understand this further, let’s evaluate the combustion of a simple fuel, methane gas. In the presence of oxygen, fuel provided with sufficient heat results in combustion. As seen in Equation 1 the complete combustion of methane (CH4) results in the products of carbon dioxide (CO2) and two molecules of water vapor (H2O).Water has an immense ability to cool the environment while suppressing or extinguishing a structure fire, thereby protecting the firefighters and trapped occupants. The practice of delaying or withholding the application of water due to the thought that it could increase water vapor and thus injure firefighter and trapped occupants was not seen in the experiments conducted. In fact, delaying water may actually increase the potential injury of firefighters and trapped occupants. Focusing on early application of water, using the reach of the stream may provide the most benefit.
References
R. Zevotek, K. Stakes & J. Willi. Impact of Fire Attack Utilizing Interior and Exterior Streams on Firefighter Safety and Occupant Survival: Full Scale Experiments, Underwriters Laboratories Firefighter Safety Research Institute. January 2018.
About the Author

Robin Zevotek
Robin Zevotek is a Principal Fire Protection Engineer with the Technical Services department at the National Fire Protection Association (NFPA) where he provides technical expertise in emergency response and responder safety as well as fire protection and building life safety. He holds both bachelor’s and master’s degrees in Fire Protection Engineering from the University of Maryland. With over 20 years experience in fire and emergency services, and extensive work in fire engineering and research, he provides a unique blend of technical knowledge and practical experience. In addition to his work at NFPA, he continues to gain practical experience and apply his expertise as the fire chief with the Ellicott City Volunteer Fire Department in Howard County, MD.